THE CLINICAL TRIAL
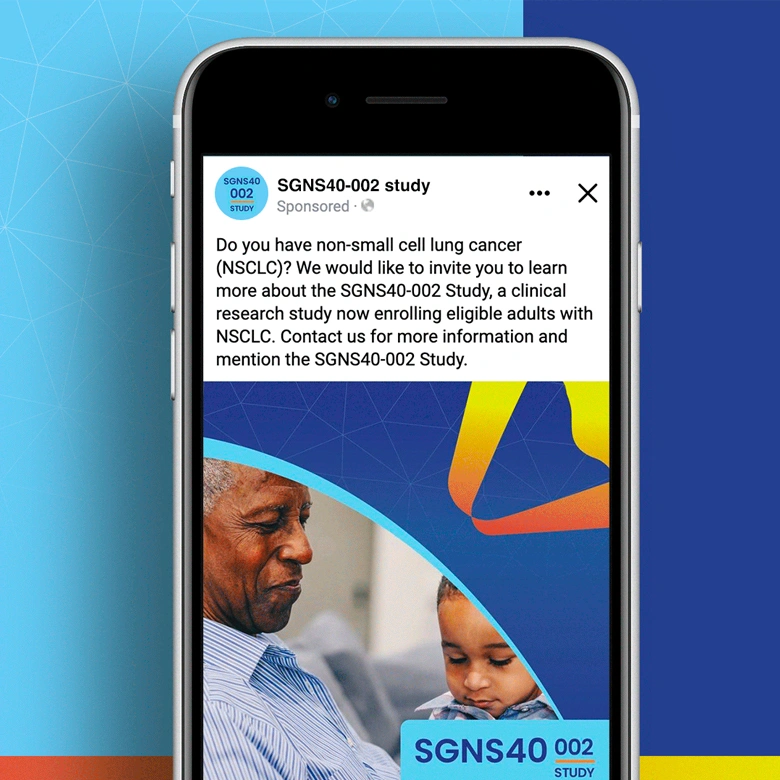
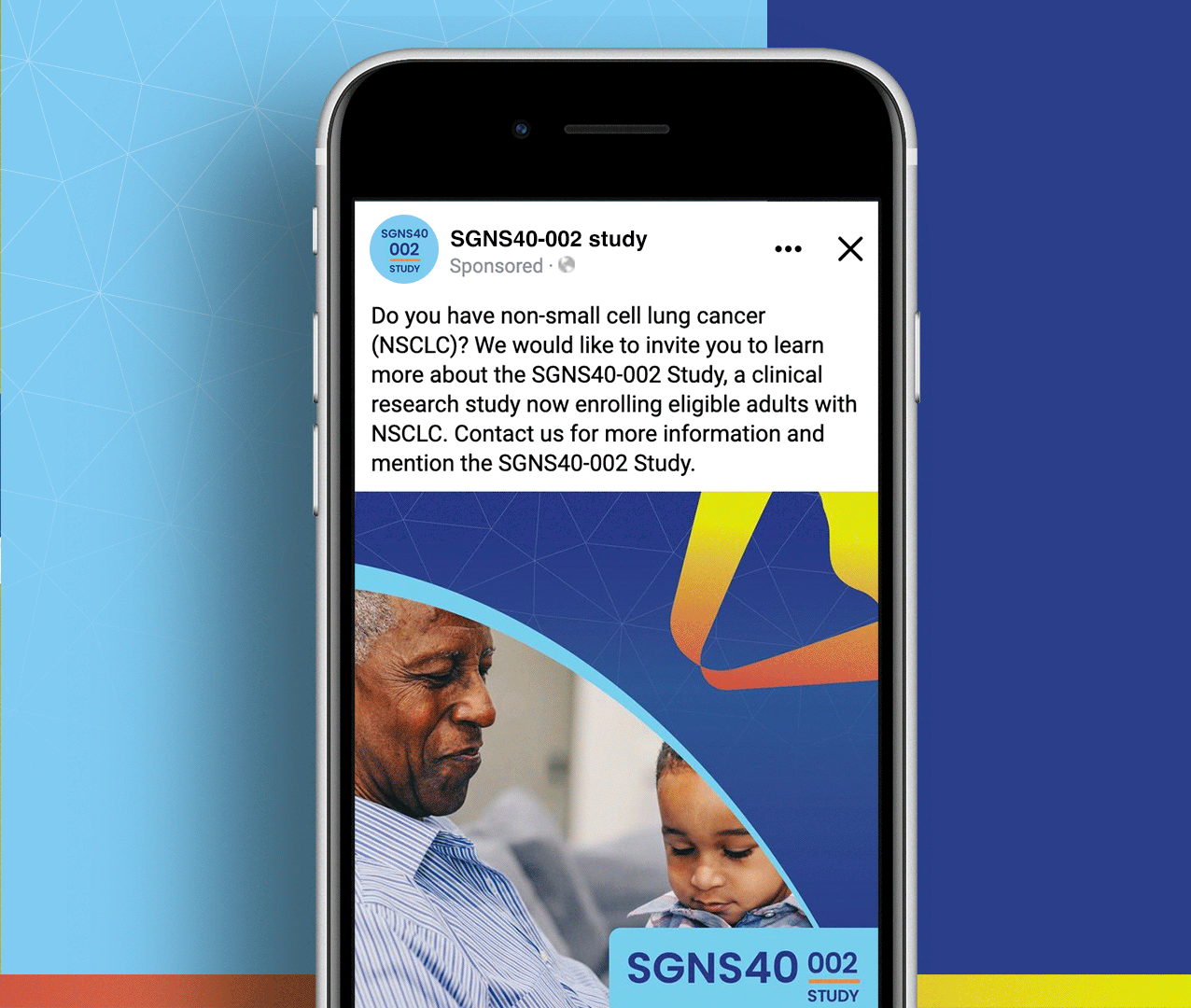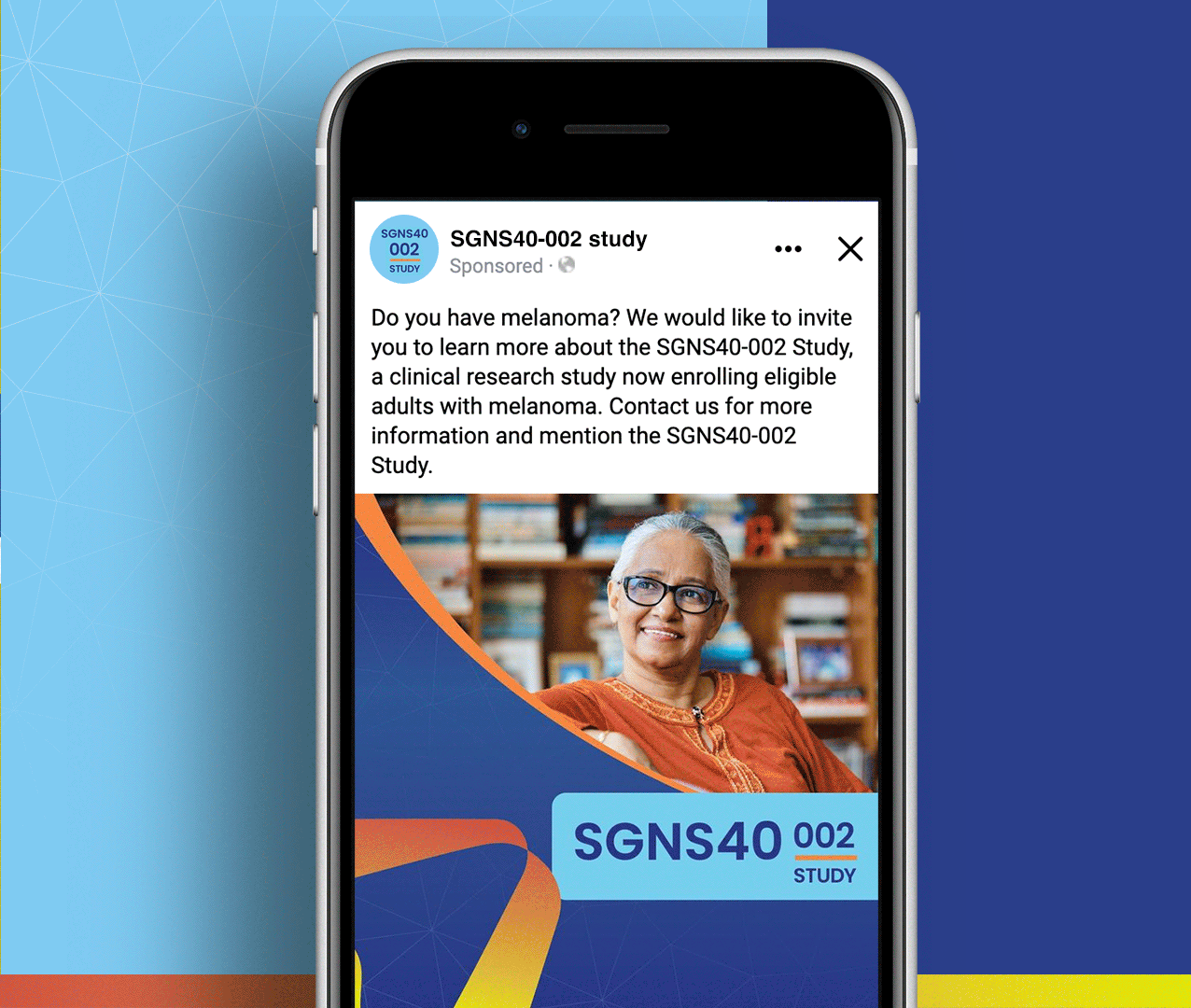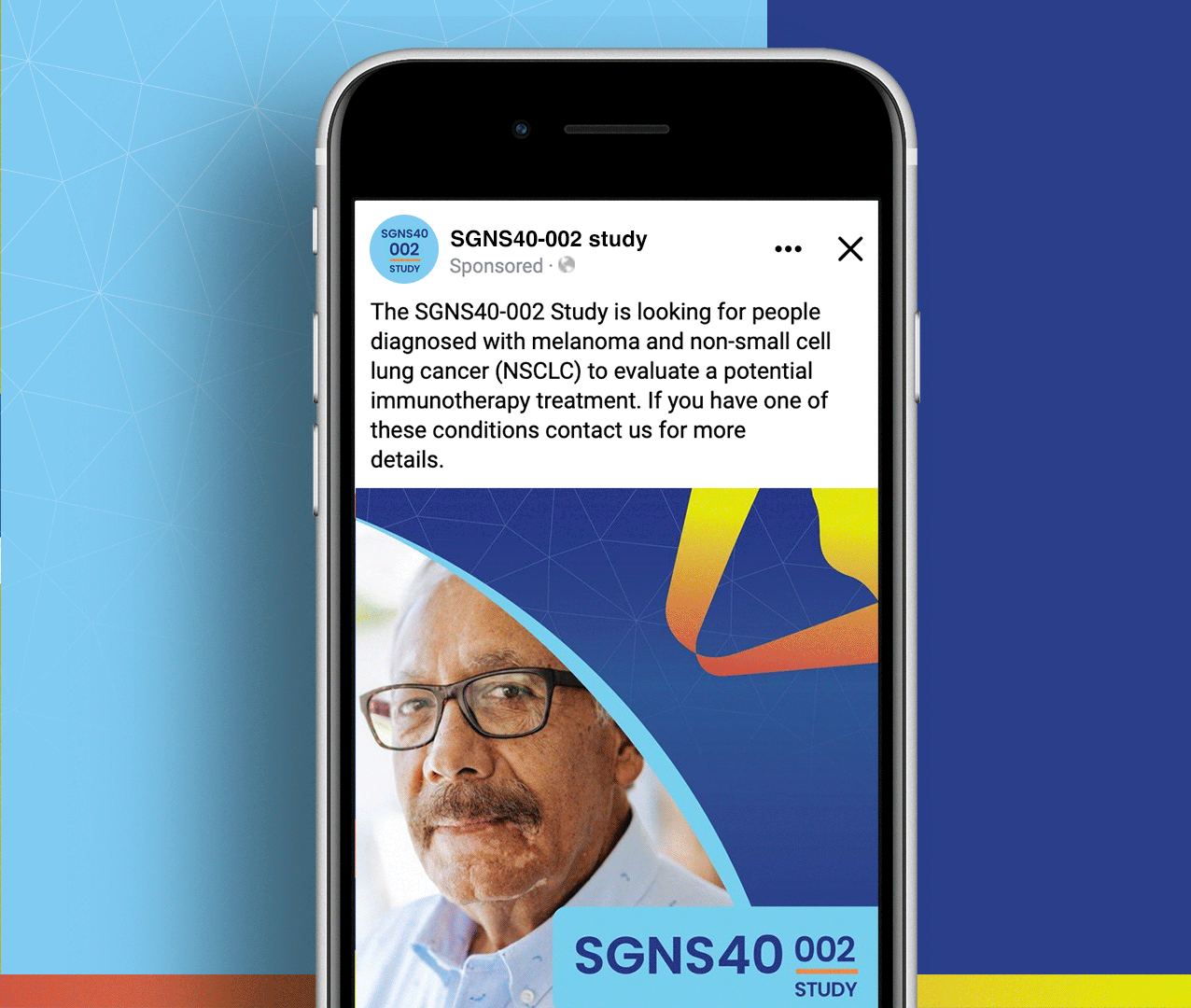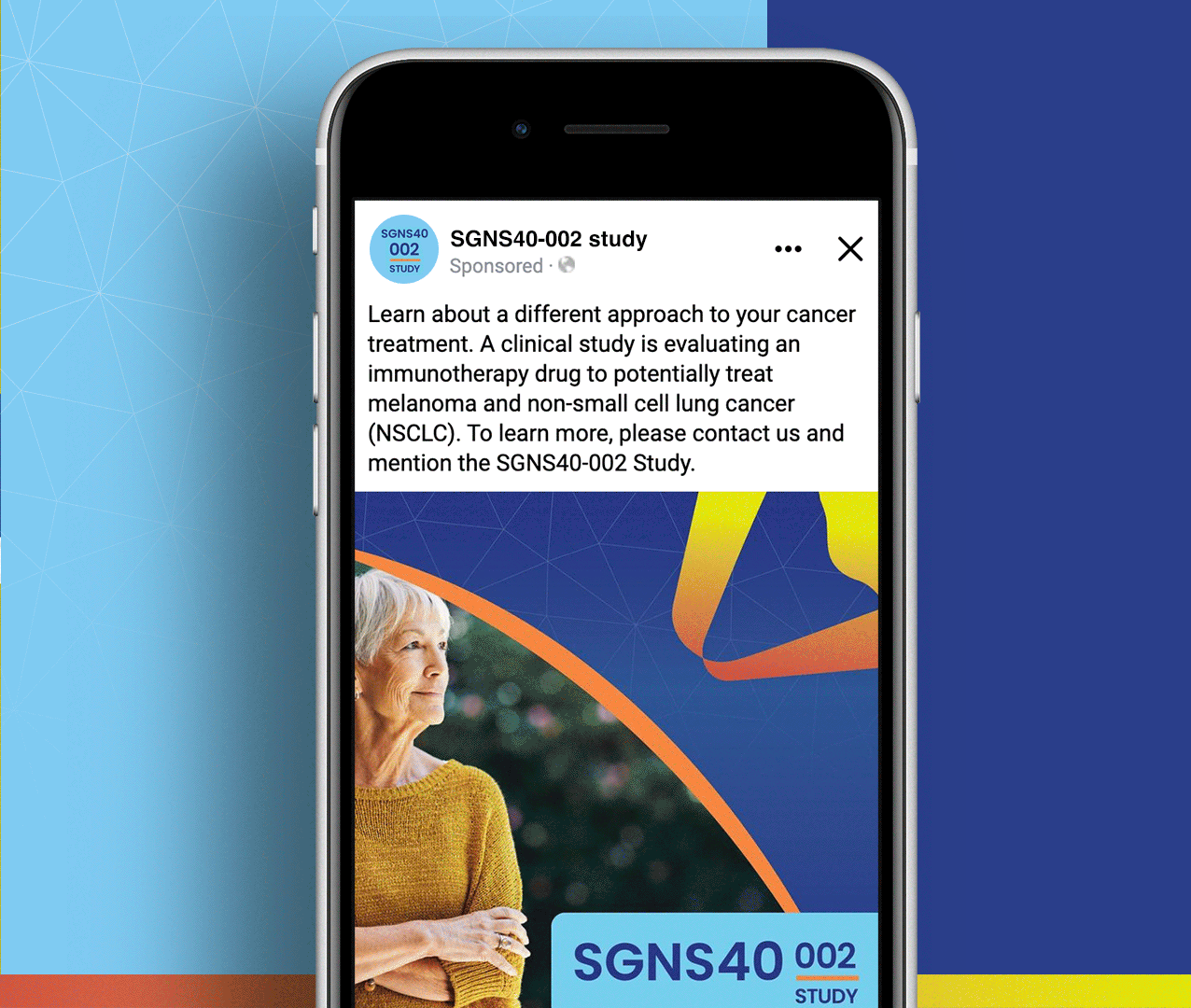
SGNS40-002 was a phase 2, open-label, multicenter trial designed to assess the antitumor activity, safety, and tolerability of SEA-CD40 in combination with pembrolizumab and/or chemotherapy in adults (≥18 years) with NSCLC or melanoma. Five indication-specific cohorts explored two different regimens: cohorts 1–3 received SEA-CD40 with pembrolizumab while cohorts 4 and 5 received SEA-CD40, pembrolizumab, carboplatin, and pemetrexed.
THE PROJECT
Seagen’s SGNS40-002 clinical team needed assistance with patient recruitment and retention for their clinical trial that included seven countries and ten languages. To achieve this, the team collaborated with Stark / Raving to create a suite of patient and site-facing materials that promoted study enrollment and facilitated study participation for two cohorts.