THE CHALLENGE
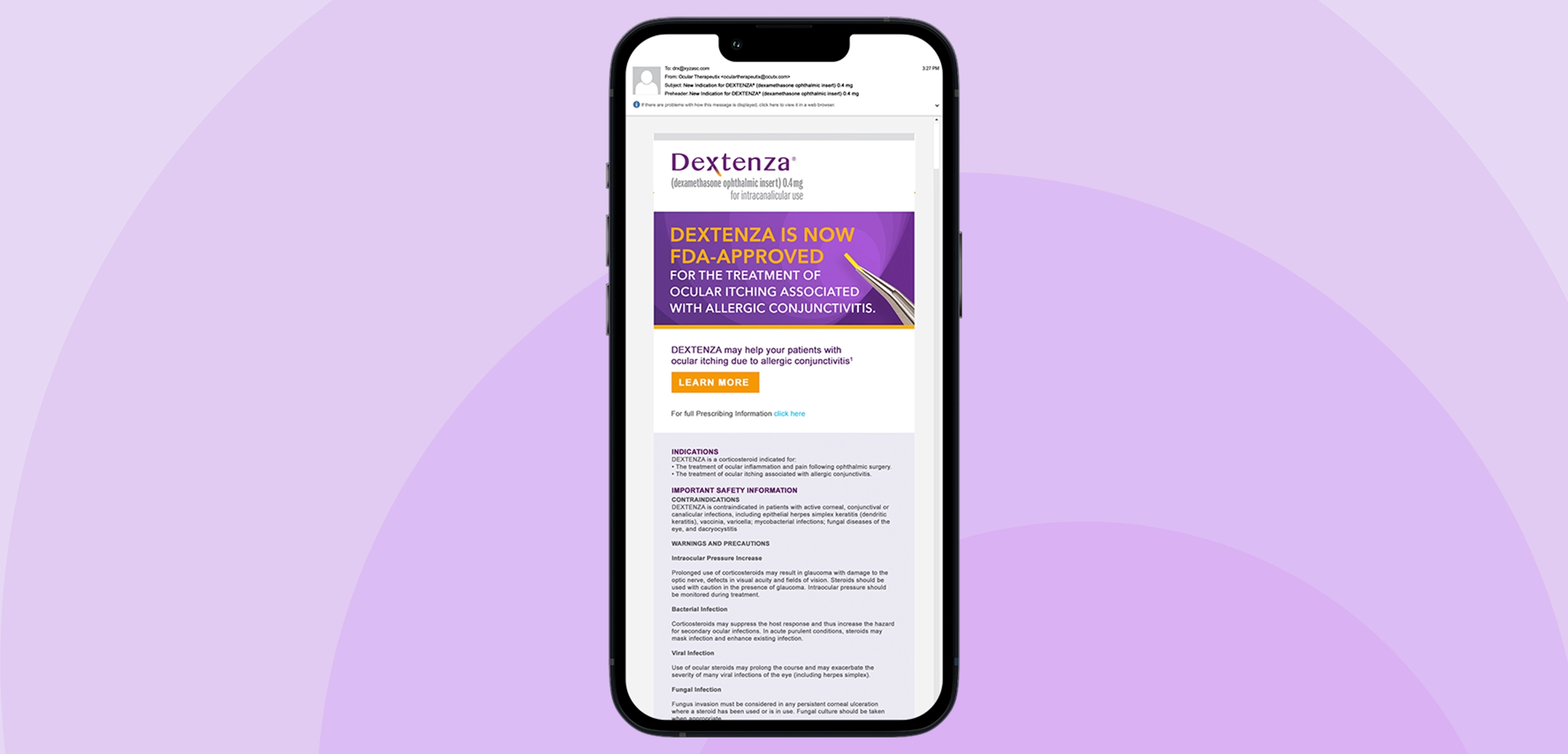
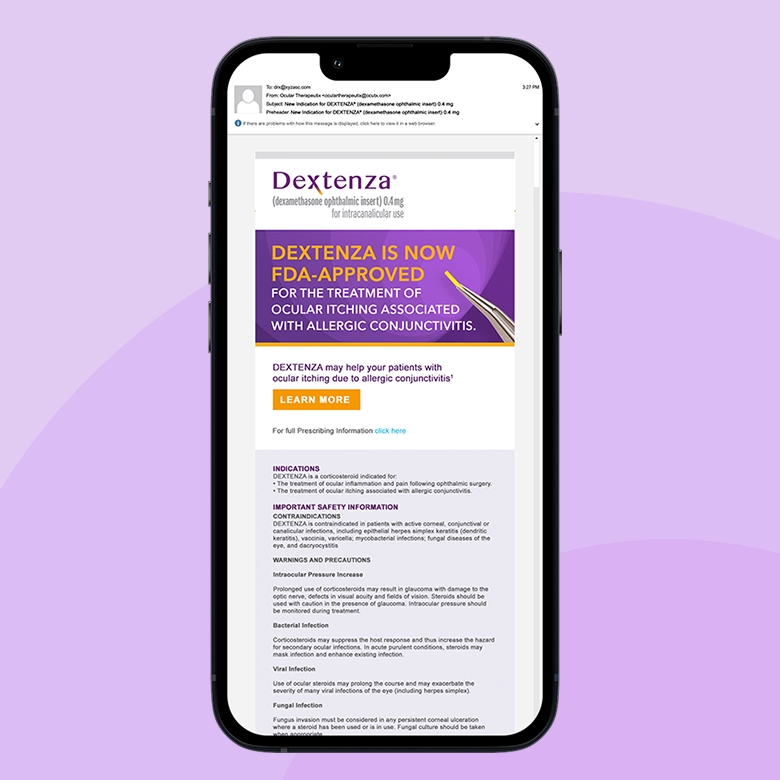
Eye surgeons knew DEXTENZA as the game-changing solution that eliminated the nightmare of post-operative eye drop compliance – one tiny insert replacing 70 individual drops and the anxiety of wondering if patients were actually following their medication regimen. But now Ocular Therapeutix had secured FDA approval for an entirely new indication: treating the misery of allergic conjunctivitis. The challenge was expanding physician understanding beyond surgical applications to recognize DEXTENZA as the solution for patients suffering from seasonal allergies who were tired of constant eye drops and limited relief.
THE SOLUTION
We repositioned DEXTENZA from a post-surgical convenience into the comprehensive solution for ocular inflammation across multiple conditions. Rather than launching a separate campaign for the new indication, we demonstrated how DEXTENZA’s proven mechanism could transform treatment for both surgical recovery and allergic conjunctivitis – giving physicians one powerful tool to address their patients’ most frustrating eye care challenges. Our integrated offline and digital campaign reached ophthalmologists and optometrists at the moment they were treating allergy patients, showing them how one insert could eliminate patient compliance issues while delivering superior relief. The result: successful market expansion that positioned DEXTENZA as the definitive solution for ocular inflammation, regardless of the underlying cause.