THE CHALLENGE
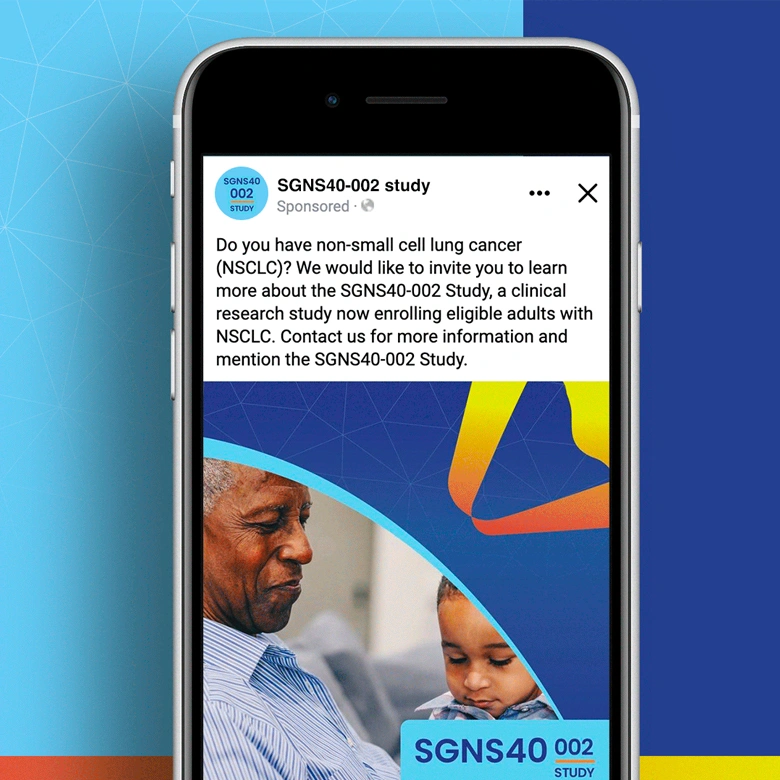
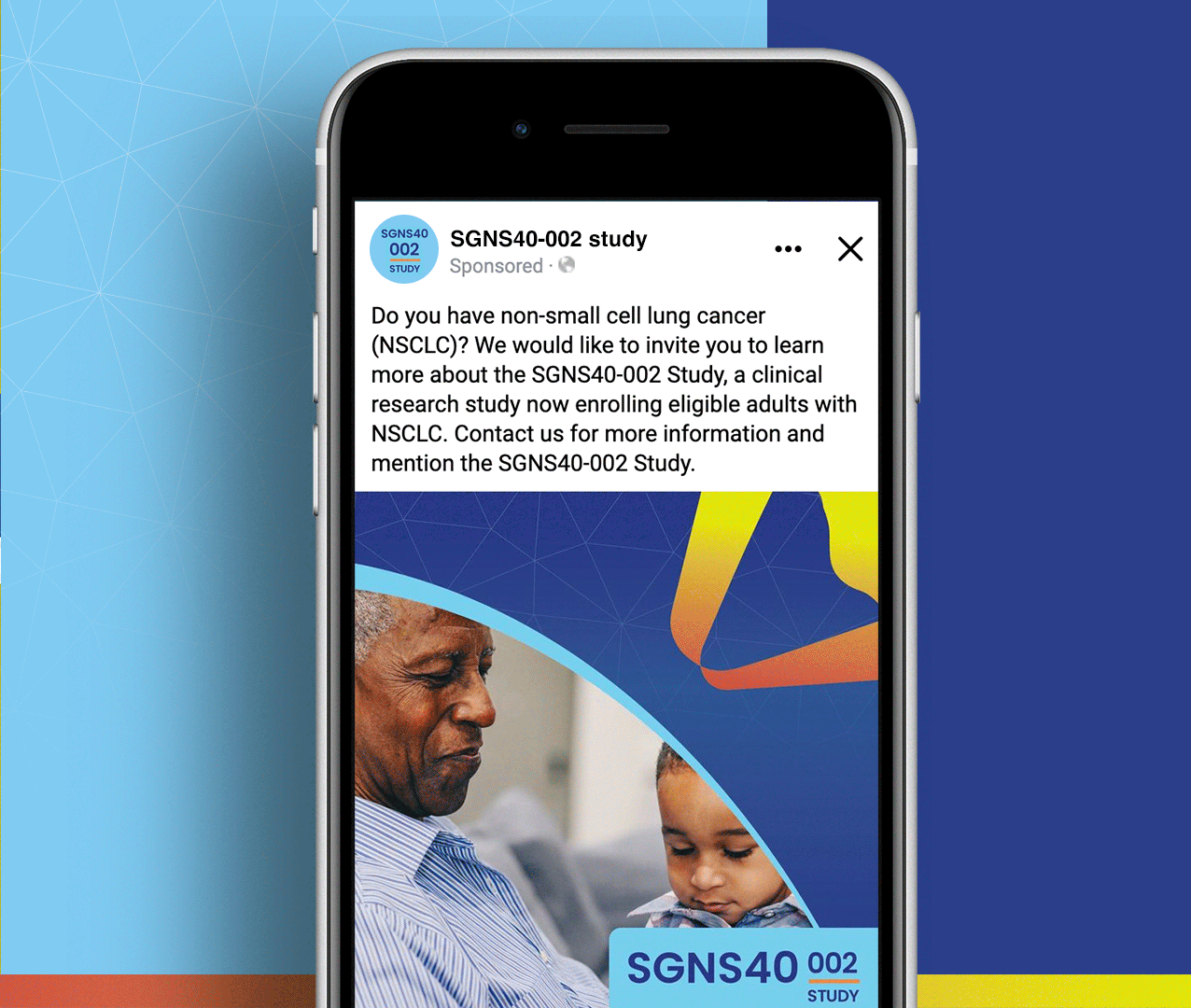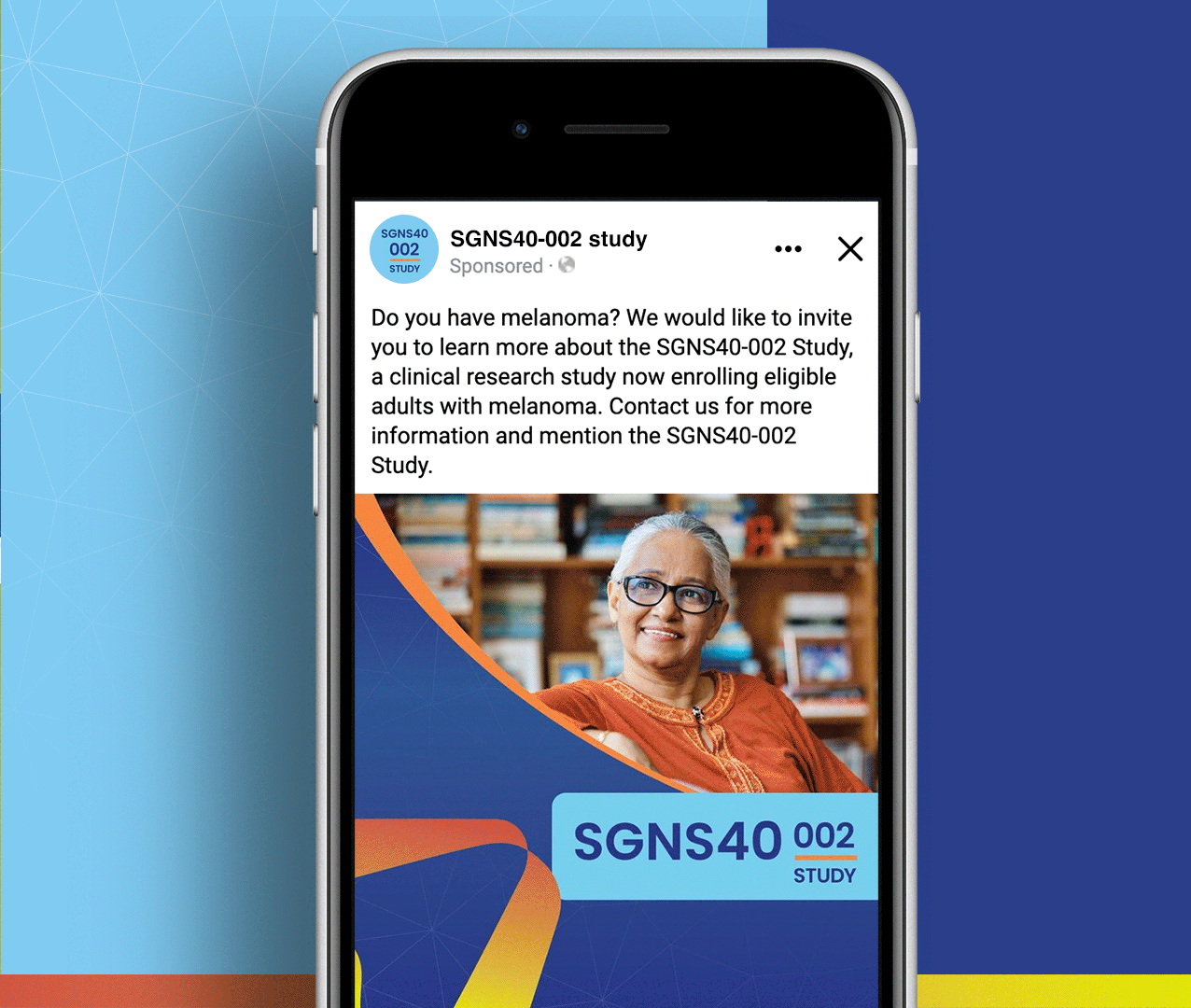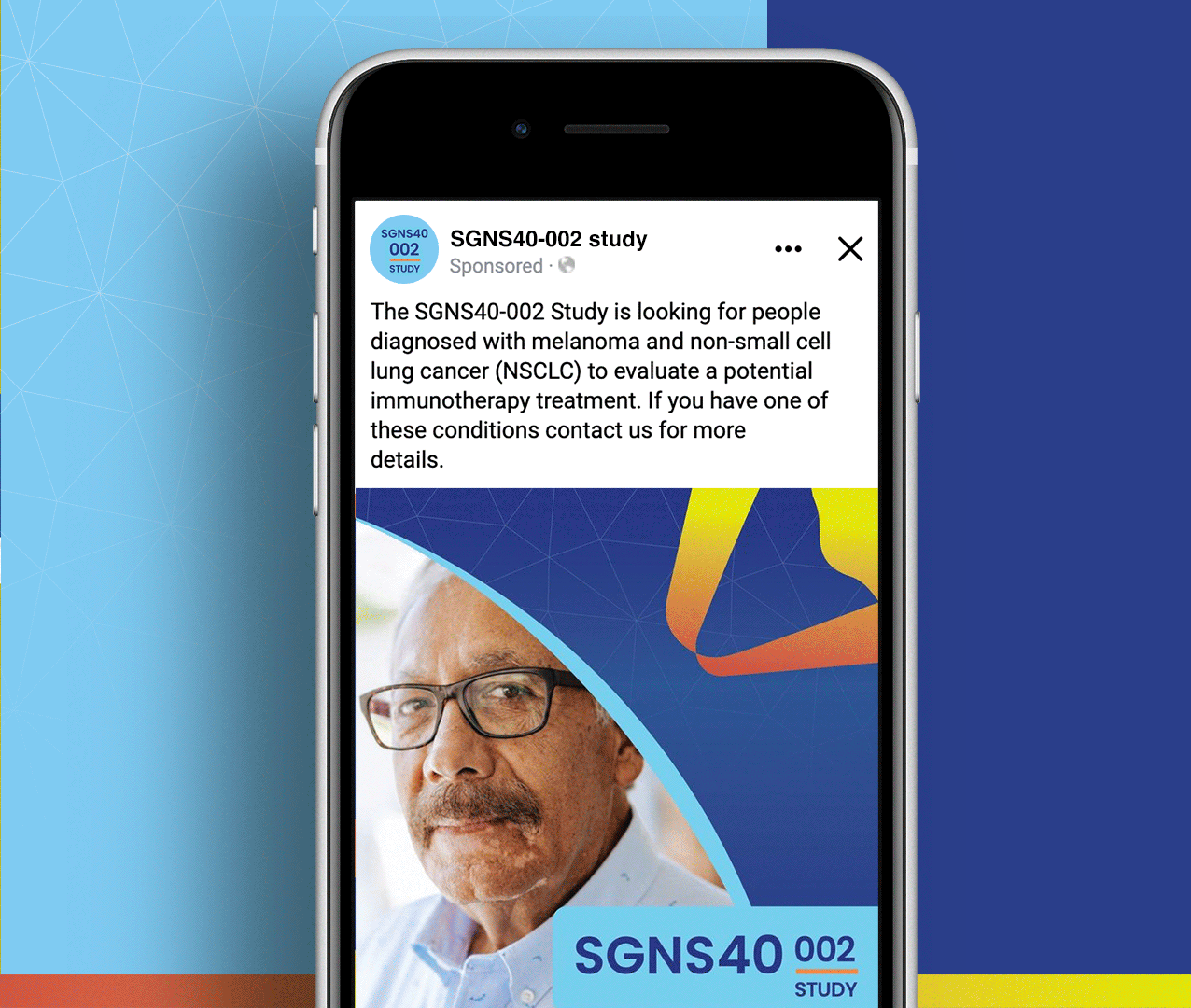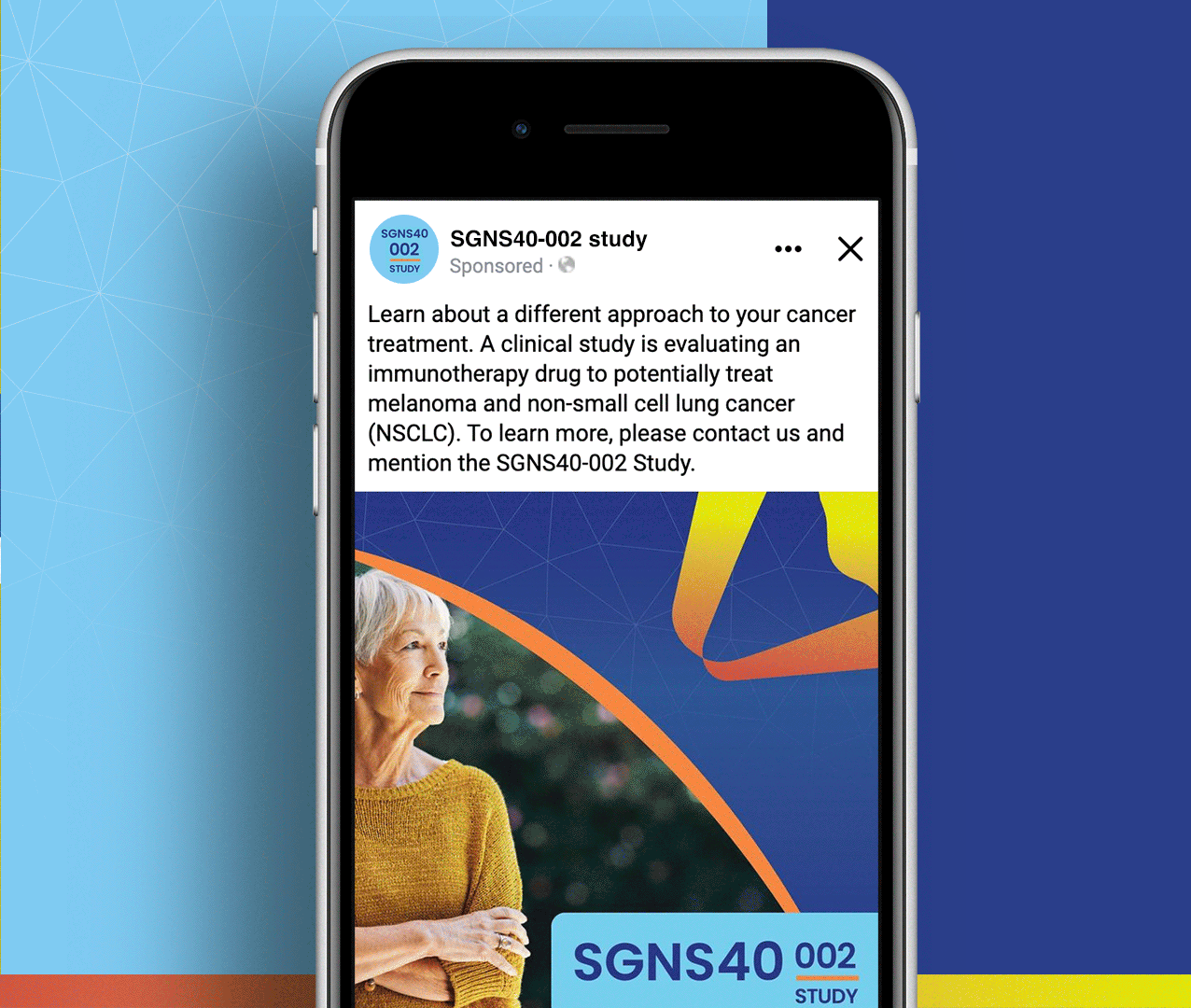
Non-small cell lung cancer and melanoma patients were running out of treatment options, but Seagen had developed SEA-CD40, a revolutionary immunotherapy that could potentially supercharge the body’s own immune system to fight these aggressive cancers. However, they faced a massive global recruitment challenge: how do you reach desperate cancer patients across seven countries and ten languages while managing the complexity of five different treatment cohorts with specific eligibility requirements? The stakes couldn’t be higher—patients needed access to breakthrough treatments, but complex international trials often struggle to enroll enough participants to prove effectiveness.
THE SOLUTION
We created a comprehensive global recruitment strategy that transformed Seagen’s complex multi-cohort trial into clear pathways of hope for cancer patients worldwide. Rather than overwhelming patients with technical details about immunotherapy combinations, we focused on what mattered most: the possibility of accessing cutting-edge treatment that could extend their lives when other options had failed. Our culturally sensitive materials spoke to patients in their native languages while helping them understand which cohort offered the best chance for their specific situation. The result: a recruitment campaign that not only filled the challenging SGNS40-002 study but also established a new standard for inclusive global cancer research that truly serves the diverse populations fighting these devastating diseases.